Sterile Water For Injection Usp Specification
I c Note on Cross-Referenced Submissions Sponsors should provide the Control Number and File Number for the submissions or sections being cross-referenced in support of the current application. BRIEFING 797 Pharmaceutical CompoundingSterile Preparations USP 41 page 6554 and PF 416 NovDec.
Lonza Water For Cell Culture Water Lonza
Revised on June 1 2019 USP received.

Sterile water for injection usp specification. After publication of the. In accordance with the Rules and Procedures of the 20152020 Council of Experts USP is postponing the official date of Pharmaceutical CompoundingSterile Preparations. For sterile products that are repackaged for blinding purposes it should be demonstrated that sterility is maintained.
Medical devices produced under standard manufacturing conditions in accordance with the requirements for quality management systems see for example ISO 13485 might prior to sterilization have. A sterile medical device is one that is free of viable microorganisms. Based on the number and significance of public comments received in response to the revision proposal published in PF 416 the USP Compounding Expert Committee is.

Sterile Water For Injection Usp Ph 5 0 7 0

Water For Injection Fda Prescribing Information Side Effects And Uses

Iv Fluid Solution Bags For Iv Therapy Iv Fluids Iv Therapy Medical Equipment Storage

Sterile Water For Injection Usp Ph 5 0 7 0

Water For Injection Usp Sterile Grade Intermountain Fisher Scientific

Water For Injection Usp Sterile Grade Intermountain Fisher Scientific

Sterile Water For Injection Usp Ph 5 0 7 0
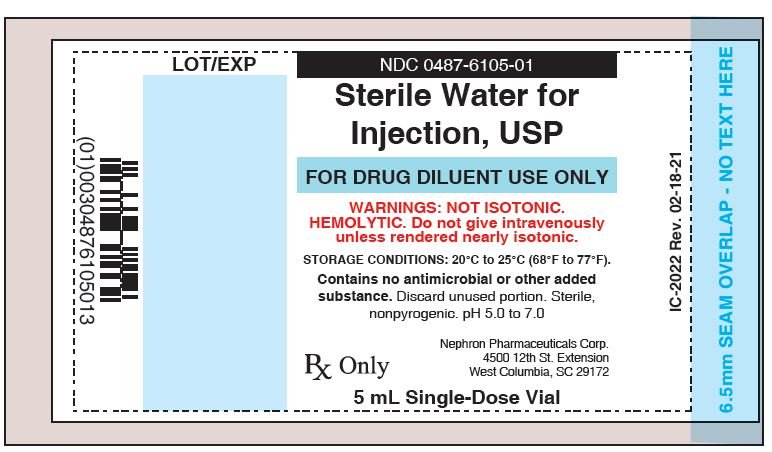
Sterile Water For Injection Fda Prescribing Information Side Effects And Uses
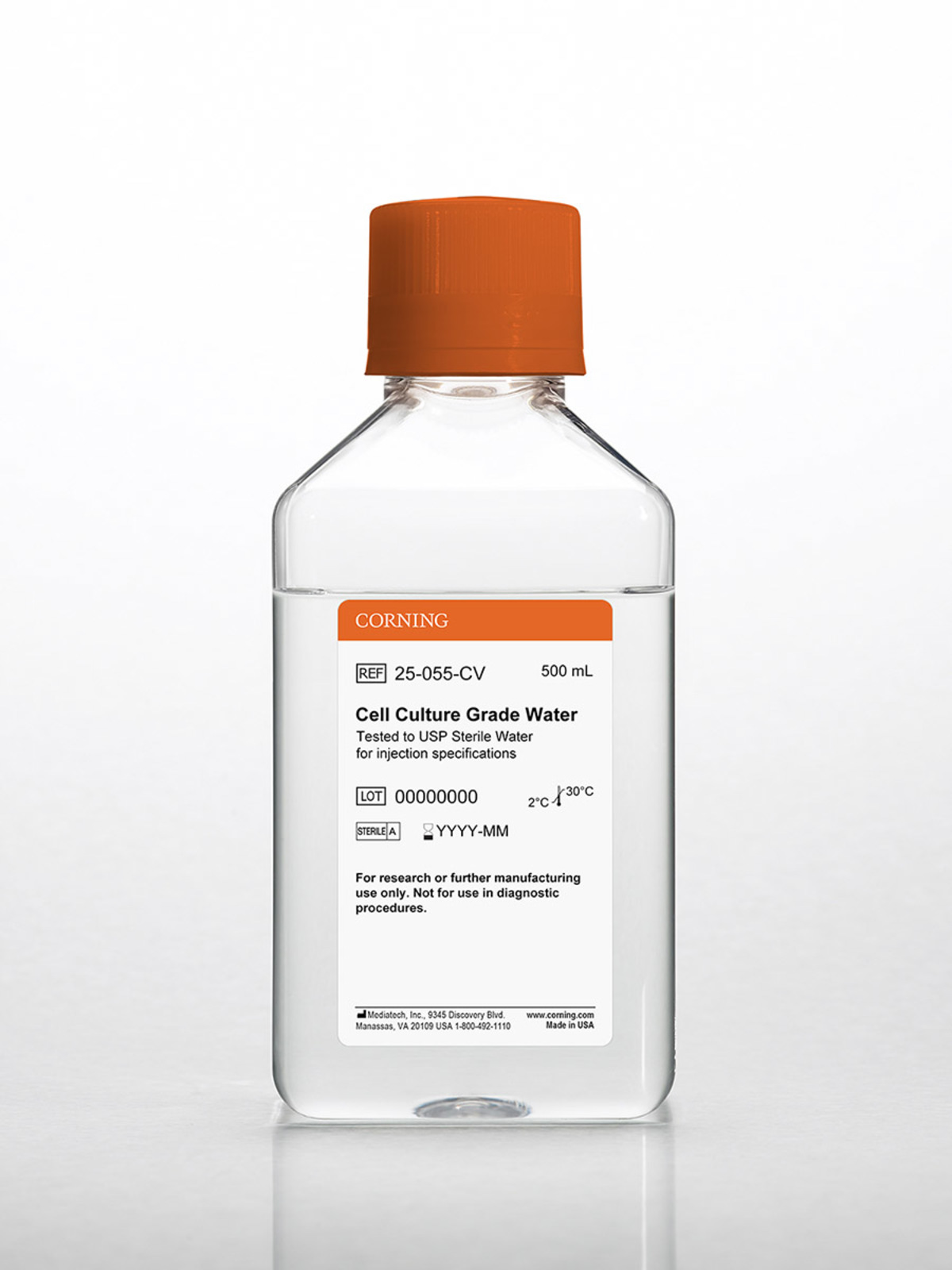
Corning 500 Ml Cell Culture Grade Water Tested To Usp Sterile Water For Injection Specifications Cell Culture Grade Water High Quality Water Media Sera And Reagents Life Sciences Germany Consumer Site Corning

Sterile Water For Injection Usp Teligent Canada

Water For Injection Wfi Usp Grade Sterile

Water For Injection Usp Sterile Grade Intermountain Fisher Scientific

Water For Injection Wfi Quality Water

Water Cell Culture Grade Tested To Usp Specifications For Sterile Water For Injection Vwr

Sterile Water 11oz Aerosol Spray Veltek Usp Wfi Water Filtered At 0 2 Microns Va Vai Wfi Sp 11z Cleanroom World

Water For Injection Usp Sterile Grade Intermountain Fisher Scientific

Sterile Water For Injection Usp

Sterile Water For Injection Usp Intermountain Life Sciences Vwr

Water For Injection Usp Sterile Grade Intermountain Fisher Scientific
Posting Komentar untuk "Sterile Water For Injection Usp Specification"