Water For Injection Specification Ep
This guideline applies to human and veterinary medicines. Manufactured under cGMP and ISO 13485 guidelines in a FDA registered facility.

Water For Injection At Thomas Scientific
Water for injections in bulk is obtained from water that complies with the regulations on water intended for human consumption laid down by the competent authority or from purified water.
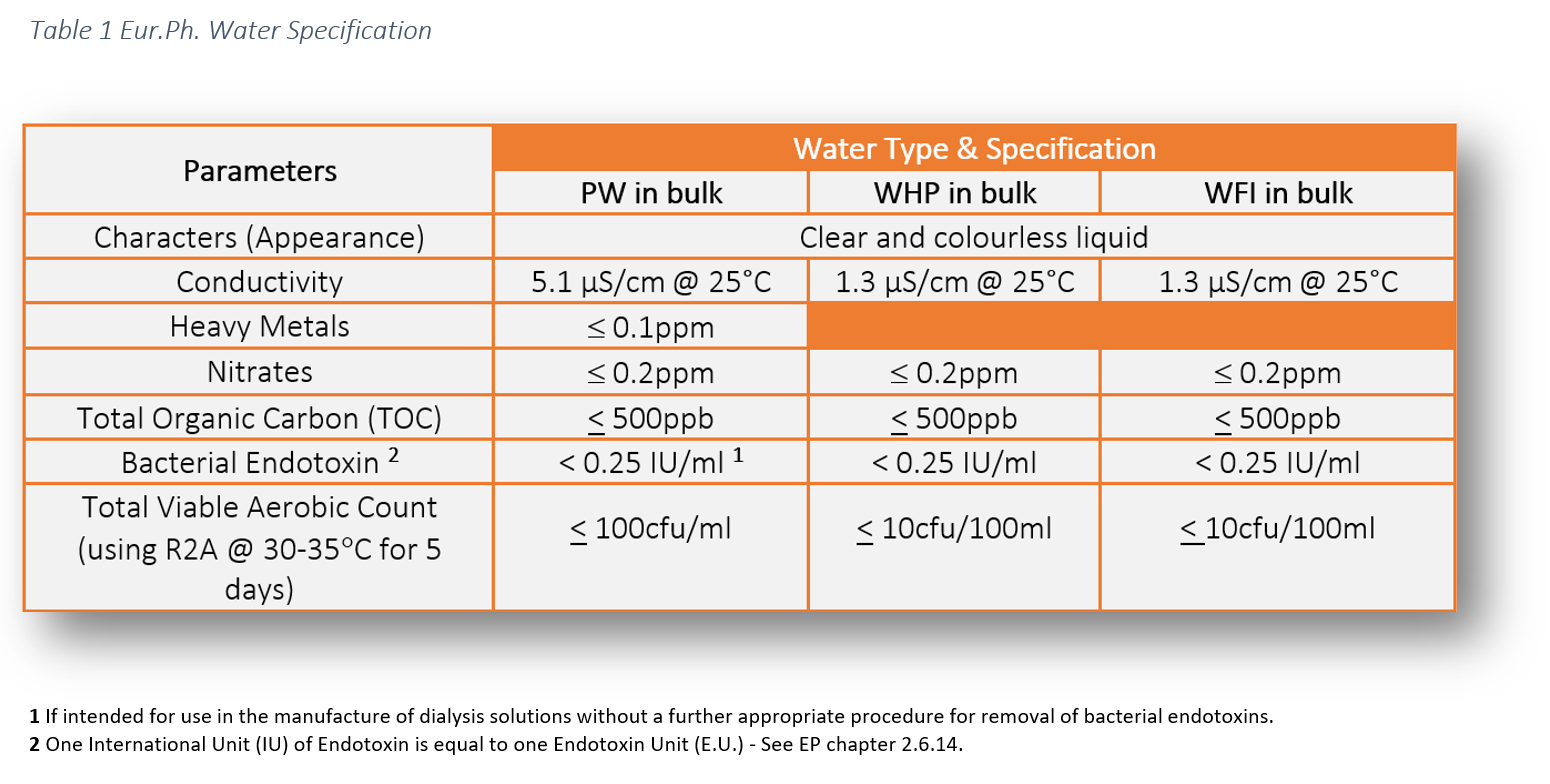
Water for injection specification ep. The guideline has also been updated to reflect current expectations. Cell culture grade water goes through a wide array of testing which includes testing to the chemical and physical properties found in the USP and EP monographs for sterile Water for Injection WFI. The flexible container is fabricated from a specially formulated non-plasticized film.
86 Water for Injections 87 Purified Water 88 Water for preparation of extracts 8941. It must meet the same chemical specification as PW but a much higher C Microbial. Our WFI-Quality Water is not to be confused with Sterile Water for Injection which is intended for use in extemporaneous prescription compounding and as a diluent for injectable parenteral products.
ICH Q3C does not apply to existing commercial drug product. Water for Injections is hypotonic and should not be administered alone. EUUnit of measurement for endotoxin activity.
Rapidly circulating water in a purified water system will control the formation of bacterial biofilms. This products resides on a Fisher Scientific GSA or VA contract. The twist-off cap is a one-time use tamper evident feature and the.
PH 50 to 70. PW must meet the chemical specification for conductivity Total Organic Carbon TOC and microbial specification. USP bacteriostatic water for injection USP sterile water for irrigation The USP designation means that the water is the subject of an official monograph in the current US.
The European Pharmacopoeia chapter on TOC for PW and WFI EP 2244 calls for total oxidation of the organic molecule for accurate TOC analysis so if some of the carbon atoms remain bound into. Please confirm that the USP requirement applies to all existing commercial drug products. Other grades of water 82 5.
Water storage and distribution systems 84 7. Water for Injection Sterile Water for Injection and Sterile Water for Irrigation have an allowable endotoxin limit of 025 Endotoxin Units EUml. We manufacture and test our Water For Injection WFI-quality products to strict industry standards.
EP specifications in the loop. Water quality specifications 77 41. Our WFI-Quality Water is not to be confused with Sterile Water for Injection which is intended for use in extemporaneous prescription compounding and as a diluent for injectable parenteral products.
Action Levels in USP 100cfumL for Purified Water and 10cfu100mL for Water for Injection are generally considered to represent a level above which the water is unfit for use. Do not use for intravenous injection unless adjusted to approximate isotonicity with a suitable solute. WFI is water of a higher purity.
Complies with the USP and EP monographs for water for injection packaged in bulk for commercial use. Water for Injection EPUSP is a terminally distilled sterile non-pyrogenic preparation which contains no bacteriostat antimicrobial agent or added buffer. Water for Injection USP is chemically designated H 2 O.
Requirements of the European Pharmacopoeia 85 The European Pharmacopoeia provides quality standards for the following grades of water. General considerations for water purification systems 83 6. It is produced either-by distillation in an apparatus of.
Water for Injection must be produced by distillation. That is why an OOS investigation must be undertaken if those Action Levels are exceeded. Undergoes extensive purification to meet or exceed the stringent specifications of United States Pharmacopeia USP Water for injection WFI is produced in ISO 9001 certified facilities to ensure a high quality product.
There are considerable differences between the EP and USP chapters on elastomers and the test for rubber closures in the JP 25. Water storage and distribution systems 78 61 General 78 62 Materials that come into contact with systems for water for pharmaceutical use 78 63 System sanitization and bioburden control 80 64 Storage vessel requirements 80 65 Requirements for water distribution pipework 81 7. Bulk purified water 80 44.
Good practices for water systems 85 8. USP sees no reason to exclude product from the requirements as the goal is to limit residual solvents in all products. Exceeding the bacterial count level of less than 10 CFU per 100 mL for water for injection will result in bacterial endotoxin contamination.
Containing polypropylene and thermoplastic elastomers free flex bag. Here the EP and the USP are already closely aligned in their tests and specifications following the revision of the USP chapter in 2008 2324. Sterile Water for Injection is a pharmaceutic aid vehicle and parenteral fluid replenisher after addition of an appropriate solute.
The guideline has been updated to reflect changes in the European Pharmacopoeia including the revised monograph for Water for Injections allowing methods other than distillation for producing water of injectable quality. 55 Production of water for injections 77 6. The plastic vials feature a twist-off cap which when removed allows access to a luer-lock fitting for connection to a luer-lock syringe.
Actual test results on a CoA are not taken from system samples but must be from actual packaged samples of the given lot. Potable Water 90 Potable Water is not covered by a pharmacopoeial monograph but must comply with the regulations on. Bulk water for injections 81 45.
Sterile Water for Injection USP in VIAFLEX Plastic Container For Drug Diluent Use Only DESCRIPTION Sterile Water for Injection USP is sterile nonpyrogenic distilled water in a. In international pharmacopeia namely Purified Water PW and Water For Injection WFI. When Water for Injections is used as diluent of hypertonic solutions appropriate dilution should be applied to bring the solution close to isotonicity.
Water for Injection USP is chemically designated H2O. 56 Dead Legs shall be measured by the term LD where L is the leg extension from the inside diameter wall normal to the flow pattern or direction and D is the inside diameter of the extension or leg of a tubing fitting or the nominal dimension of a valve or instrumentNon-final product water pipingtubing in the PWWFI Pretreatment and Production. Pharmacopoeial specifications 78 42.
The plastic single-dose vial is fabricated from polypropylene resin. Our WFI-Quality Water is aseptically processed from a validated Water For Injection System that meets current USP and EP specifications in the loop.

Revised Wfi Guidelines Offer Greater Choice Of Techniques

Water For Injection Ep Usp Sterile Grade Intermountain Fisher Scientific

Water For Injection Ep Usp Sterile Grade Intermountain Fisher Scientific

Jim Knows Best Pharmaceutical Waters Industry Expert Mettler Toledo

Water For Injection Ep Usp Sterile Grade Intermountain Fisher Scientific

Water For Injection Ep Usp Sterile Grade Intermountain Fisher Scientific
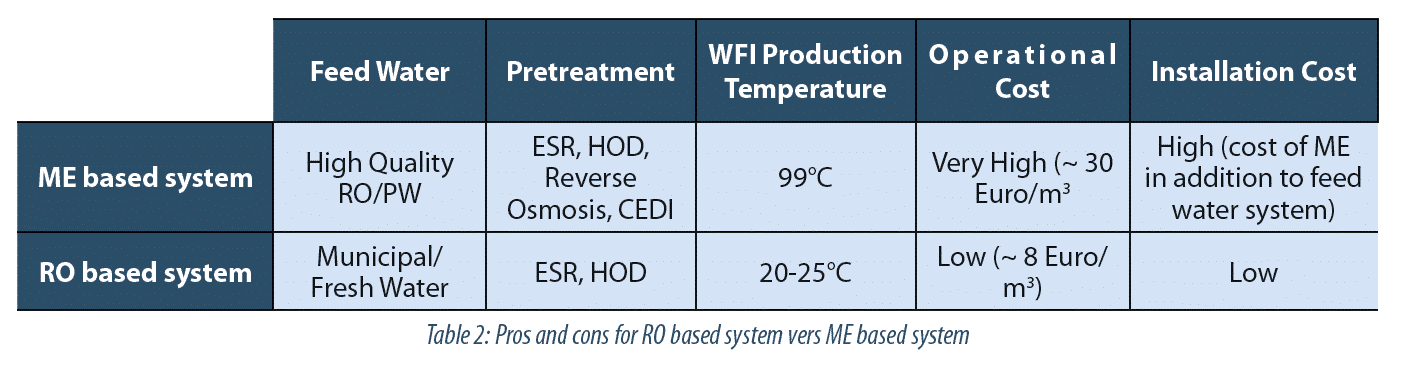
Comparison Of Wfi Production By Membrane Based Method Distillation Based Method According To The Revised Ep Monograph For Wfi Production A3p Pharmaceutical Biotechnology Industry

Water Usp Purified Sterile Filtered Wfi Quality 55 Gallon Southern Labware

Water For Injection Wfi Analysis
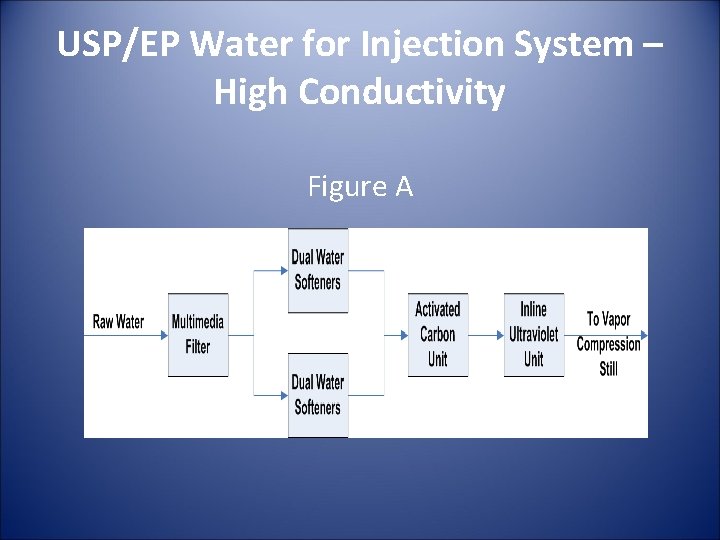
Uspep Purified Water And Water For Injection Systems

Bsb 3503 Biomanufacturing Chapter 11 Gmp Pharmaceutical Water
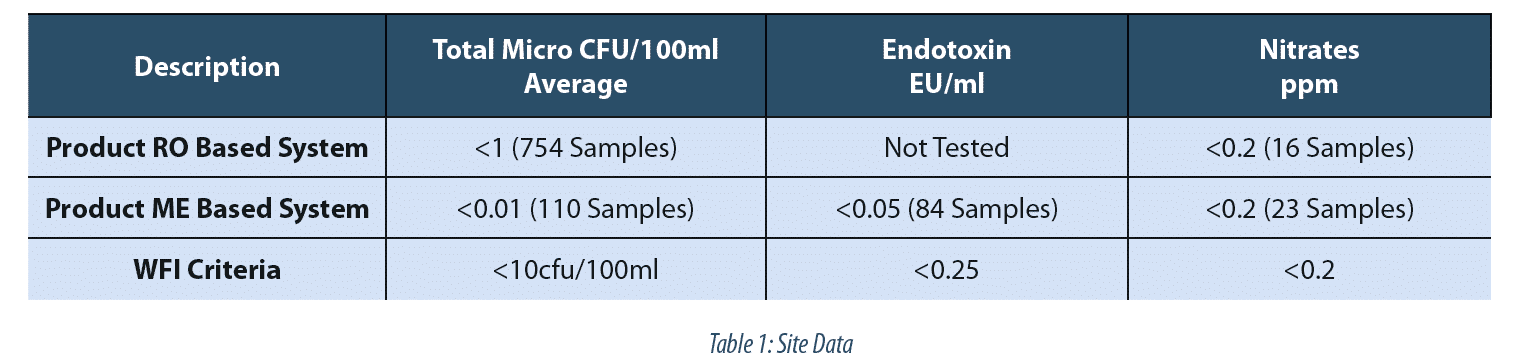
Wfi Production By Membrane Distillation Based Methods A3p

Uspep Purified Water And Water For Injection Systems

Water For Injection Ep Usp Sterile Grade Intermountain Fisher Scientific
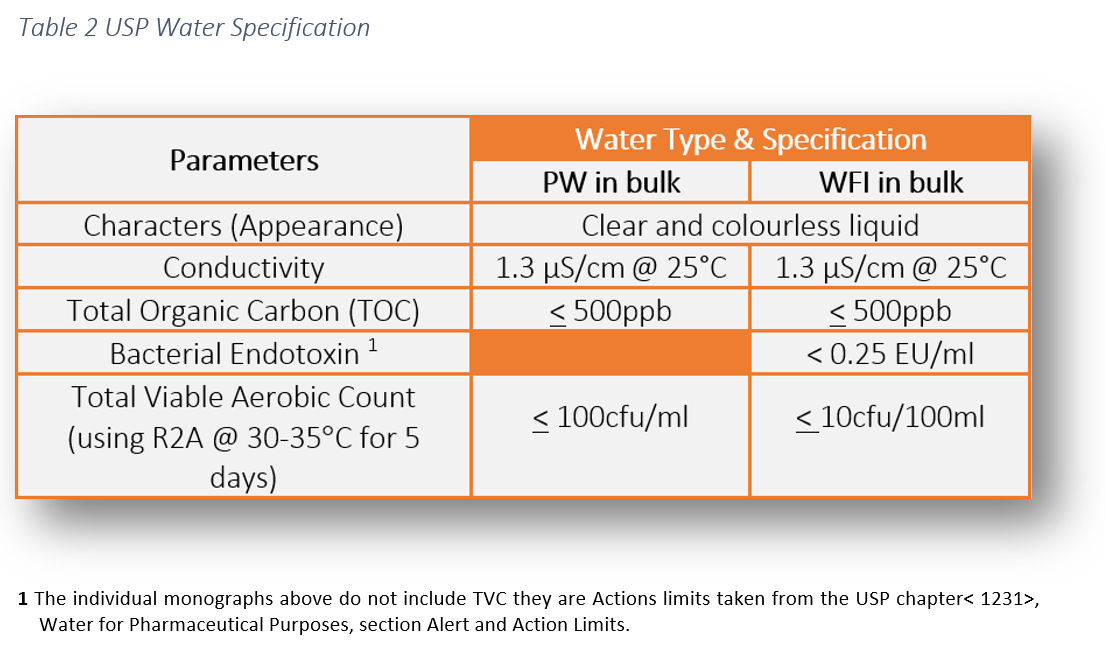
Water For Injection Wfi Analysis

Water For Injection Ep Usp Sterile Grade Intermountain Life Sciences

Ppt Usp Ep Purified Water And Water For Injection Systems Case Histories Powerpoint Presentation Id 464025

Highly Purified Water Products 500 Ml Usultra Co
Posting Komentar untuk "Water For Injection Specification Ep"