Usp Specifications For Purified Water
Total organic carbon 643. USP Sucrose RS.
Hot Water Sanitization Ro A Plain And Simple Introduction Wcp Online
Purified Water Specification as per IPBPUSP.
Usp specifications for purified water. Purify water to meet USP 23 specifications. Also know what is USP grade water. 55 WFI is the most demanding and expensive to produce and is generally used when necessary eg.
It is produced either-by distillation in an apparatus of which the parts in contact with the water are of neutral. SAFETY DATA SHEET 1. USP sterile water for irrigation The USP designation means that the water is the subject of an official monograph in the current US PHARMACOPEIA with various specifications for each type.
Stock Up On Your Favorites Today. The procedure described below is designed for measuring the conductivity of Purified Water and Water for Injection. Ad Order today with free shipping.
USP Sterile Purified Water. 1927-1929 and Water for Pharmaceutical Purposes p. 3 USP grade waterStandard USP water specifications for pharmaceuticals manufacturing are conductivity 0210 μScm at 25C.
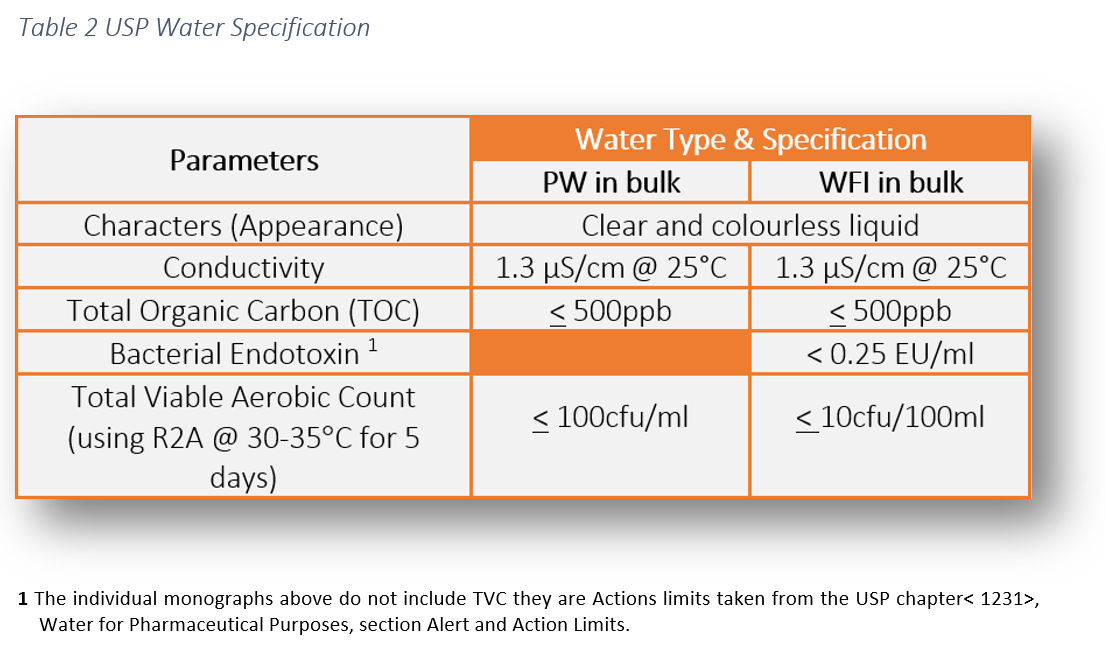
Action Levels in USP 100cfumL for Purified Water and 10cfu100mL for Water for Injection are generally considered to represent a level above which the water is unfit for use. Purified Water PW Normally the. Properties USP Purified Water USP Water for Injection Highly Purified Water Conductivity µScm 25C Total Organic Carbon TOC ppb or µgL Bacteria guideline Endotoxin EUml 13 500 100 cfuml NA 13 500 10 cfuml 025 EUml.
TOC level 500 ppb. 20 rows We have established a process purified water charcoal treatment softening UV sanitization. This is USP Purified Water that has been sterilized and suitably packaged.
That is why an OOS investigation must be undertaken if those Action Levels are exceeded. And bacteria count 100 cfu100 ml. GuidelinesWater Supply Parameters page 3 and below Fresh Water Systems Inc.
Permit production by. Get the Deals now. SPECIFICATION Purified Water Ph.
USP Standards for Packaged Purified Water Water for Injection and Sterile Purified Water USP24 effective 1100. The fol-lowing point of use specification is summarized in the following table. USP24 contains complete versions of all pharmaceutical water monographs p.
USP Reference standards 11 USP 1 4-Benzoquinone RS. Warrants to the original purchaser the PharMate 4300 USP Purified Water System and PharMate Dispenser models when in materials and workmanship under normal use within the operating specifications for a period of 2 years from the date of purchase. On addition of Methyl red solution the resulting solution should be not red.
Clear Colorless odorless and tasteless liquid. Stage 1 of the procedure below may alternatively be performed with the appropriate modifications to Step 1 using on-line instrumentation that has been appropriately calibrated whose cell constants have been accurately determined and whose temperature compensation function has. Water for injections in bulk is obtained from water that complies with the regulations on water intended for human consumption laid down by the competent authority or from purified water.
Purified Water Specifications Conductivity USP 24 Specification Endotoxins No Specifications Bacteria 100 cfuml pH 50 70 TOC 500 ppb Water-for-Injection WFI Systems The components that comprise the WFI system are. Water for Injection WFI USPJP permits distillation or a purification process that is equivalent or superior to distillation in the removal of chemicals or. 52201231 Water for Pharmaceutical Purposes General Information First Supplement to USP 35NF 30 DBP levels in drinking water can be minimized by using Purified WaterPurified Water see the USP monograph disinfectants such as ozone chloramines or chlorine diox-is used as an excipient in the production of nonparenteral ide.
WFI also requires bacteria count 10 cfu100 ml and endotoxin level 025 EUml 74. USP Standards Contaminant Parameter and Unit Type 1 Type 2 Type 3. 1752-1754 and the general chapters TOC Water Conductivity p.
Tested to USP Purified Water Specifications No toxic agents including DEPC are used in the preparation of reagent grade water eliminating possible interferences with enzymatic reactions Corning reagent grade water is high-quality water which is manufactured. Also what is purified water used for. All Spectrum Chemical USP grade products are manufactured packaged and stored under current Good Manufacturing Practices cGMP.
Sterile Purified Water is often used when access to a USP Purified Water generating system is not possible or practical. Purified Water packaged in bulk for commercial use elsewhere meets the requirements of all of the tests under Sterile Purified Water except Labeling and Sterility 71. For the final purification steps of parenteral products.
Replacing the heavy metals attribute was considered unnecessary because a the source water specifications found in the NPDWR for individual Heavy metals were tighter than the approximate limit of detection of the Heavy metals test for USP XXII Water for Injection and Purified Water approximately 01 ppm b contemporary water system. Distillation reverse osmosis de - ionization filtration or equivalent means. SPECIFICATION TYPICAL RESULT Appearance EP Clear colorless liquid Pass Total Organic Carbon1 USPEP.
2154-2163We can not provide photocopies of copyrighted materialAmmonia mgl. Ad Shop Our Variety of Purified Water in Bulk Online. 50 to 70Chloride mgl.
219 current version It is the users responsibility to ensure fitness for use of this packaged article when it is used in manufacturing clinical or analytical applications where the purer bulk form of. Specification for Purified Water USP W1014 Item Number W1014 Item Purified Water USP CAS Number 7732-18-5 Molecular Formula H 2O Molecular Weight 1802 MDL Number Synonyms Deionized Water Test Specification Min Max Total Organic Carbon TO PASS TEST Water Conductivity TO PASS TEST. Shop Anywhere At Anytime Boxed Provides The Premium Delivery Service Right To Your Door.
Pharmacopoeias like EP USP WHO. On addition of Bromo thymol blue solution the resulting should not blue. The United States Pharmacopoeia USP has three general specifications for water quality that are applicable to medical and pharmaceutical uses namely USP Water for Injection WFI USP Purified Water and Drinking Water.
The minimum requirements for USP Pure Steam are those found in EPAEUWHO Drinking Water standards. Purified Water USP has gone through a purification process to attain a level of purity.
Water For Injection Wfi Analysis
Uspep Purified Water And Water For Injection Systems
Usp Standards To Purified Water And Water For Injection Wfi Download Table
Purified Water Standard Requirements Download Table
Corning 500 Ml Molecular Biology Grade Water Tested To Usp Sterile Purified Water Specifications Molecular Biology Grade Water High Quality Water Media Sera And Reagents Life Sciences Asia Pacific Business Site Corning
Purified Water Products 500 Ml Usultra Co
Water For Injection Wfi Analysis
Purified Water Water For Injection Sop As Per Usp Pharmawiki In
Purified Water Standard Requirements Download Table
New Iso 22519 Pw Wfi Production Standard Three Principles
Design And Construction Of Usp Purified Water Systems
Revised Wfi Guidelines Offer Greater Choice Of Techniques
Qualification Of Purified Water Systems Semantic Scholar
Usp Standards To Purified Water And Water For Injection Wfi Download Table
Jim Knows Best Pharmaceutical Waters Industry Expert Mettler Toledo
High Purity Water Simplified Chemical Engineering Page 1




Posting Komentar untuk "Usp Specifications For Purified Water"